Mitoplicator™ Boosts the CHO-‐K1 Growth Curve
INTRODUCTION
The Mitoplicator (fig. 1) produces nanosecond pulsed electromagnetic fields that are transferred by an antenna into a cell culture vial and the cells in a non-‐invasive way. Like in 3T3.L1 cells1 and Hybridoma2, nanosecond pulses were shown to increase the cell number in CHO-‐K1 cultures by 41%±4 (n=12) over control cultures at day 3 of culture3.
In this application note, CHO-‐K1 grown in flat tubes4 cells were treated once with Mitoplicator to evaluate cell number and morphology from seeding to confluence over a 10 day period.
RESULTS
CHO-‐K1 cells were trypsinized and seeded in Flat-‐sided tubes at low seeding density and allowed to adhere overnight prior to stimulation with Mitoplicator.
A single stimulation (day 0) of adherent CHO-‐K1 cells5 with Mitoplicator leads to growth curve that displays a head start versus control cell cultures (figure 2). The Mitoplicator-‐induced increase in cell proliferation becomes noticeable at day 2 and appears most clearly at day 3, when 28% more cells were counted in treated cultures. From day 4 to day 6, both cultures grow at the same rate, maintaining a head start of the Mitoplicator-‐treated cultures of 1/2 day.
From day 6 onward, the cultures reach confluence and converge to identical cell densities (treated is 101% of control at days 9 and 10). Microscopic evaluation of the cultures did not elicit morphological differences between treated and control monolayers throughout the culture period.
CONCLUSION
A single treatment of CHO-‐K1 cells elicits a boost of cell proliferation, which is most notable 3 days after the stimulation. Thereafter, cultures grow at similar rates, maintaining the head start of the treated cultures until confluence is reached.

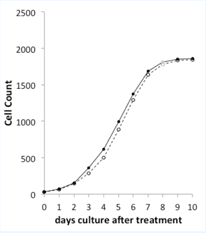
Figure 2.
Overview of the CHO-‐K1 cell growth curves for control (open symbols) & Mitoplicator treated cultures (closed symbols). The values for day 8 are an interpolation.
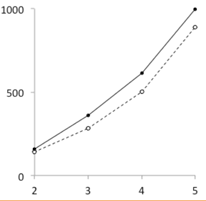
Figure 3.
Detail of the CHO-‐K1 cell growth curves (day 2 to 5).
METHODS
Cells. CHO-‐K1 (ATCC CCL-‐61) were cultured following ATCC guidelines5 in F-‐12 medium (Gibco, 21765-‐037) with 10% FBS (Gibco 10500-‐064) and 1% sodium pyruvate (Gibco, 11360-‐039). Cells were passaged twice weekly (1000 cells/cm2) and while harvested 50-‐80% confluent. 3ml of trypsinized, resuspended CHO-‐K1 cells were seeded (at 625 or 1250 cells/cm2) in 10ml tubes with a 6cm2 optically clear surface. Cells were allowed to adhere overnight prior to stimulation.
Stimulation. Prior to Mitoplicator treatment, tubes were coded. The handling before, during & after Accelerator treatment was as previously described4 and in accordance with the CHO-‐K1 ATCC culture guidelines5. Mitoplicator treatment conditions were 5 minutes at 50Hz. Following treatment, tube lids were opened and tubes placed in the incubator.
Cell counting. Monolayers were trypsinized, collected and cells were gently resuspended in medium containing 10% FBS. The number of cells was counted using a Burker-‐Turk counting chamber. Cell counting was done blind and in duplicate. The average of duplicates was recorded. Codes were broken after cell count results were recorded.
ABOUT MITOPLICATOR™ & ECPR TECHNOLOGY
The Enhanced Cell Proliferation Reactor (ECPR) technology was discovered, validated & developed by the Vabrema founders since 2009 and in close collaboration with the Eindhoven Technical University (TU/e).
Today, ECPR technology is available for R&D purposes as the Mitoplicator™ instrument and for fermenter-‐scale cell or protein production (assay/engineering project).
Proof of principles for ECPR technology includes mammalian & human cell lines, primary cells & isolates, stem cells, plant seedlings, algae. Vabrema actively pursues an R&D plan, aiming at widening ECPR applicability.
Please inquire with us if ECPR technology could be to the benefit of your R&D or company value chain.




 京公网安备 11010802034257号
京公网安备 11010802034257号